Healthcare Professionals, Adherence management has never been easier.
A clinically proven solution that meets
the needs of your largest patient group.1
With a simple to manage remote consultation model
that allows you to easily extend your therapy portfolio.
- 52% reduction in AHI amongst responders2
- 50% reduction in ODI amongst responders2
- 3.9 point reduction in ESS amongst responders2
Complete your armamentarium
eXciteOSA reduces primary snoring and mild osa by retraining the upper airway.2-5
No other device currently on the market does this.
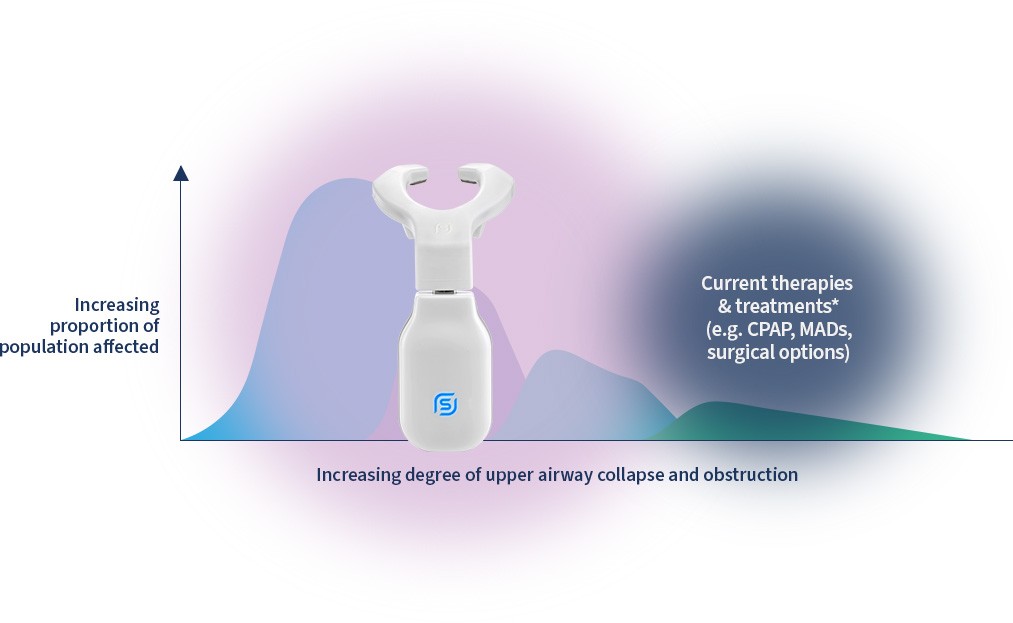
- Treat patients with primary snoring and mild OSA with eXciteOSA
- Early treatment may slow progression or possibly delay the need for other treatments
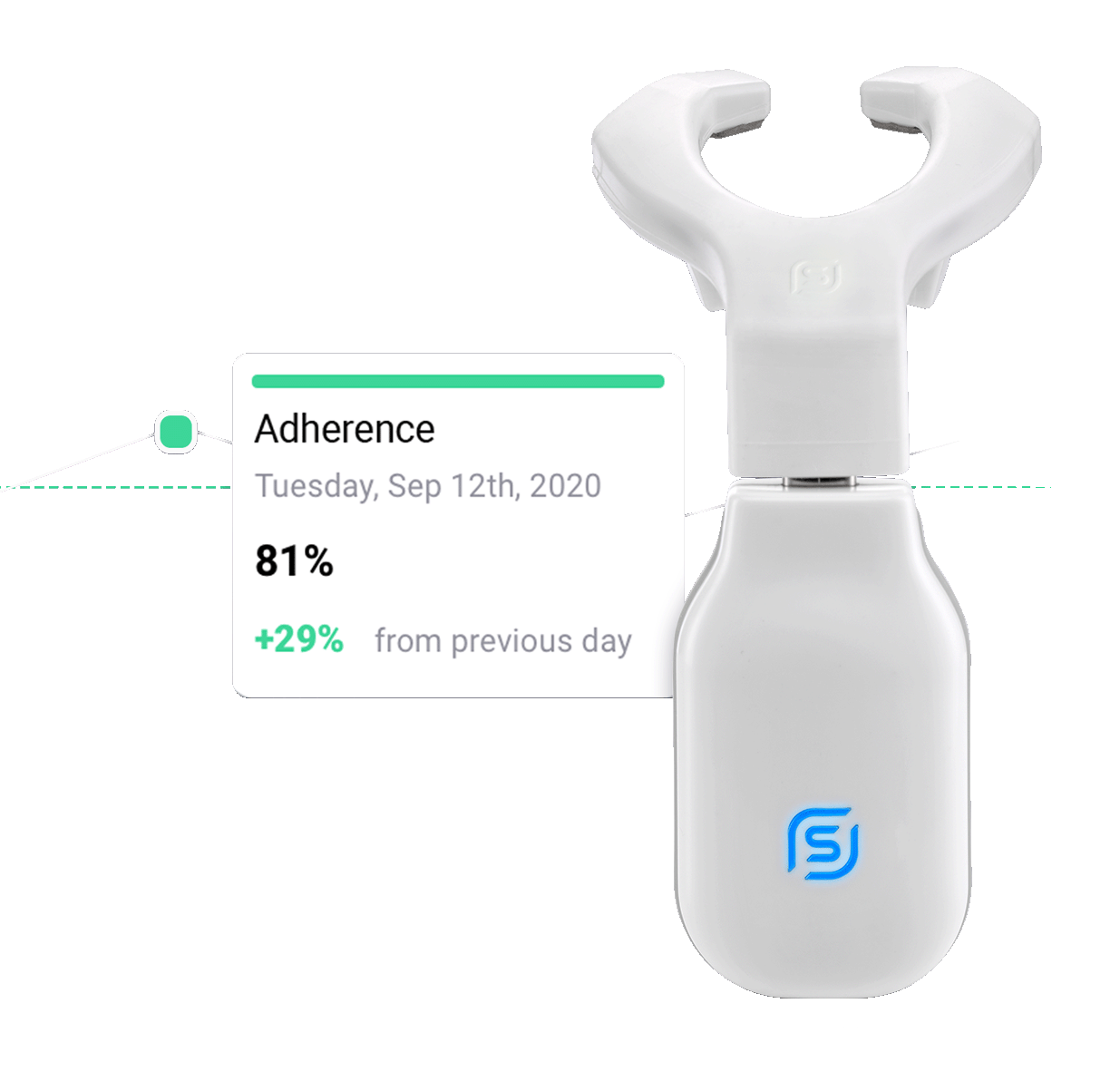
A simple way for Healthcare Professionals to manage patients
Ease of therapy + App-directed support = Patient adherence
eXciteOSA is clinically proven to reduce apneas2
A published study found a reduction of 52% in AHI and 50% in ODI amongst the patient subset who responded to therapy, all without a night-time wearable.
Healthcare Professionals can monitor and manage progress remotely
The eXcite portal allows for remote tracking to support patient adherence.
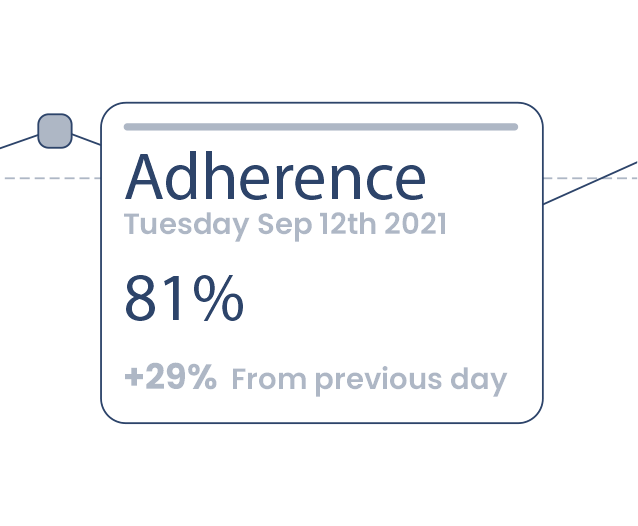
Ease of therapy encourages patient adherence
It’s easy for patients to find 20 mins in their daily schedule.
Are you ready to offer your patients a therapy that is simple, convenient and clinically proven?
Find out how easy it is to integrate eXciteOSA into your treatment continuum for primary snoring and mild osa.
If you are an existing eXciteOSA patient, please contact us here.
How It Works
eXciteOSA helps
the body help itself
By addressing muscle function, eXciteOSA is unlike traditional therapies.

Using Neuromuscular Electrical Stimulation (NMES), the mouthpiece targets and retrains the tongue in order to increase muscle endurance.
Six weeks of treatment is associated with a significant increase in endurance of the tongue muscle.7 Patients experience a noticeable reduction in symptoms after 6 weeks.2-5
The eXciteOSA Healthcare Professional Portal
Supporting and managing your
eXciteOSA patients has never been easier
Using the portal, Healthcare Professionals can easily analyze and reference their patients’
usage patterns, trends and statistics during in-person or telehealth appointments.

Valuable insights
An array of interactive reports using proprietary algorithms, highlight relevant usage insights that suit your needs.
Focused activity
The dashboard reflects real-time eXciteOSA app data, allowing you to focus only on those patients who need help.
Remote patient management
As telehealth becomes the norm and not the exception, the patient dashboard is a useful no cost tool for remote consultation and management.
Schedule a
demonstration
See how eXciteOSA will benefit your patients.
- 52% reduction in AHI amongst responders2
- 50% reduction in ODI amongst responders2
- 3.9 point reduction in ESS amongst responders2
Signifier Medical Technologies is focused on the development and commercialization of innovative solutions for patients with snoring and sleep-disordered breathing conditions.
Pioneers in challenging the wisdom of conventional snoring and sleep apnea treatments, we’ve created the first daytime therapy that tackles the root cause of sleep-disordered breathing by physiologically retraining the airway against collapse.
Newsletter sign-up
Not ready to get started, or already started? Sign up to our newsletter to be kept up to date on the many advances in NMES to treat OSA and primary snoring. We don’t spam.
References
- Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pepin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019;7:687-698.
- Nokes B, Baptista PM, Martínez Ruiz de Apodaca P, Carrasco-Llatas M, Fernandez S, Kotecha B, Wong PY, Zhang H, Hassaan A, Malhotra A. Transoral awake state neuromuscular therapy for mild obstructive sleep apnea. Sleep Breath [accepted; in-press].
- Wessolleck E, Bernd E, Dockter S, Lang S, Sama A, Stuck BA. Intraoral electrical muscle stimulation in the treatment of snoring. Somnologie 2018;22(2):47-52.
- Kotecha B, Wong PY, Zhang H, Hassaan A. A novel intraoral neuromuscular stimulation device for treating sleep-disordered breathing. Sleep Breath 2021;25(4):2083-2090.
- Baptista PM, Martinez Ruiz de Apodaca P, Carrasco M, Fernandez S, Wong PY, Zhang H, Hassaan A, Kotecha B. Daytime neuromuscular electrical therapy of tongue muscles in improving snoring in individuals with primary snoring and mild obstructive sleep apnea. J Clin Med 2021;10(9):1-11.
- Nokes B, Schmickl CN, Brena R, Bosompra NN, Gilbertson D, Sands SA, Bhattacharjee R, Mann DL, Owens RL, Malhotra A, Orr JE. The impact of daytime transoral neuromuscular stimulation on upper airway physiology – A mechanistic clinical investigation. Physiol Rep 2022;10(12):e15360.
*See For Sleep Apnea page










