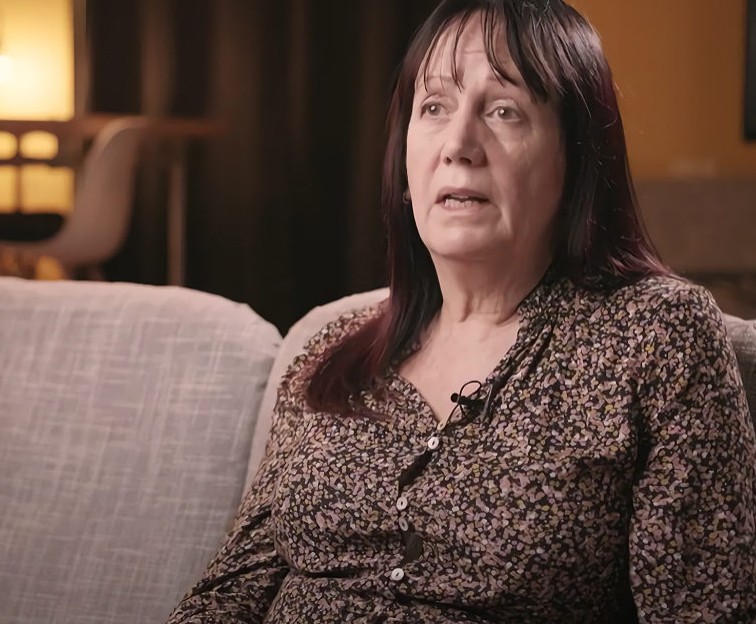Say goodbye to masks or MADs – Say hello to daytime therapy.
eXciteOSA® for sleep apnea delivers NMES therapy and has been clinically proven to reduce sleep apnea. Use for just 20 minutes a day, every day for six weeks, to improve the quality of your sleep.1-3
Clinically proven daytime therapy. Nothing to wear at night.

Treatments
There are several options for treating mild obstructive sleep apnea
Many OSA therapies focus on creating space in the airway. NMES does something unique.
1. Nighttime treatments
Uncomfortable. Difficult to tolerate
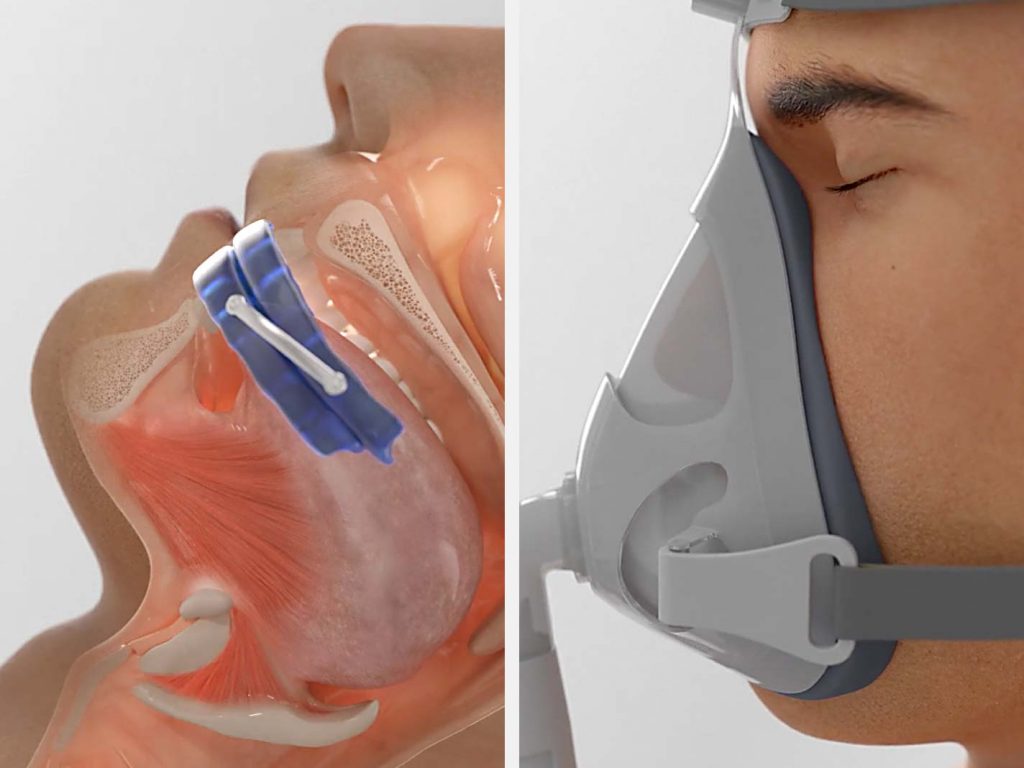
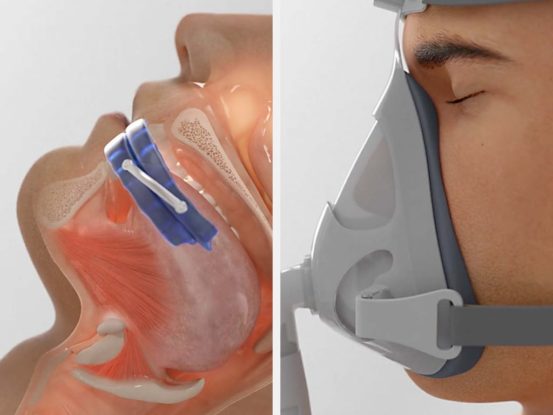
Mandibular (jaw) Advancement Devices work by temporarily moving the jaw forward. CPAP machines pump air pressure into your upper airway.
2. Surgical treatments
Invasive
Surgery is usually an option after other treatments have failed. Examples include tissue or tonsil removal.
3. Daytime NMES therapy
Simple, effective & painless
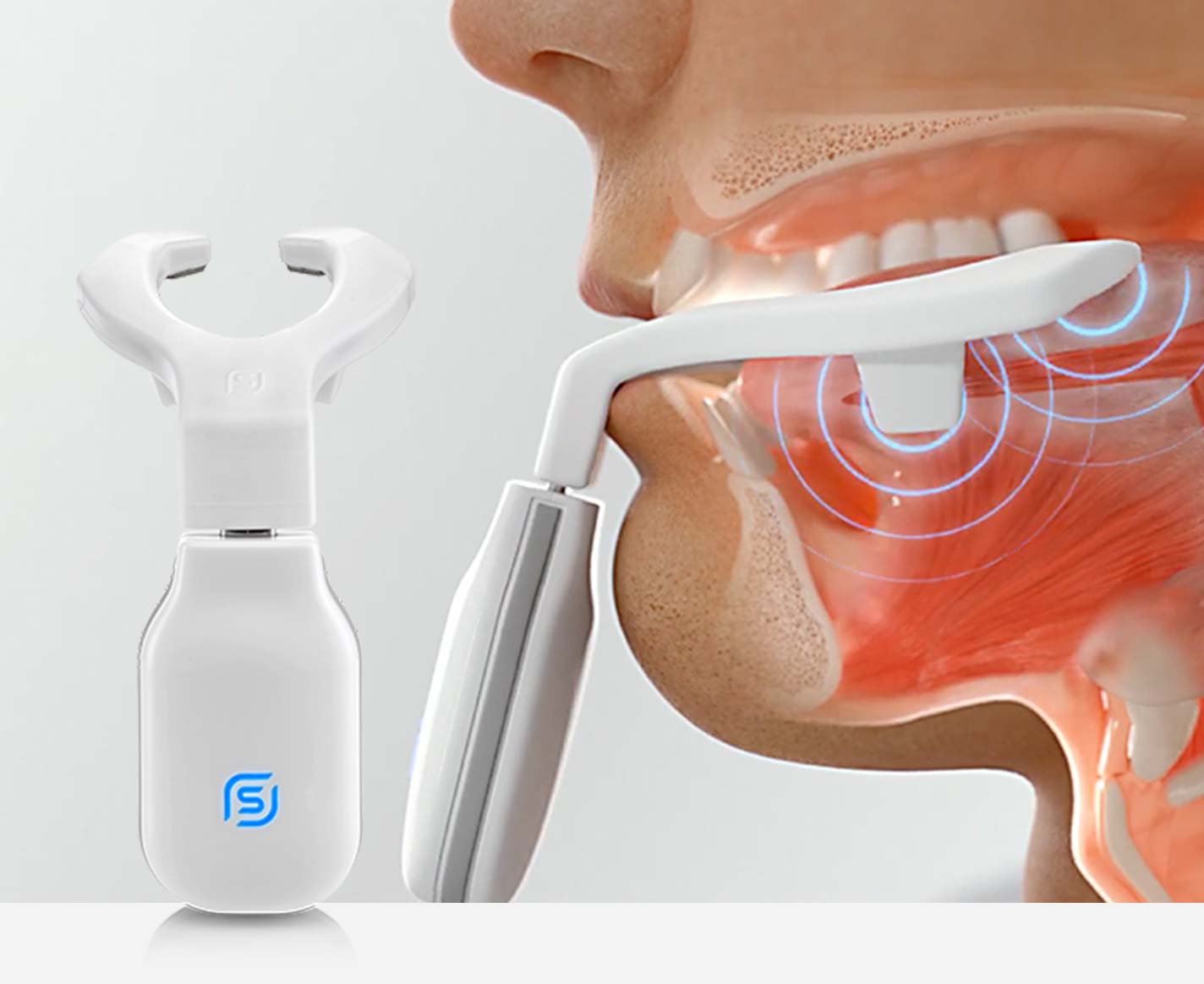
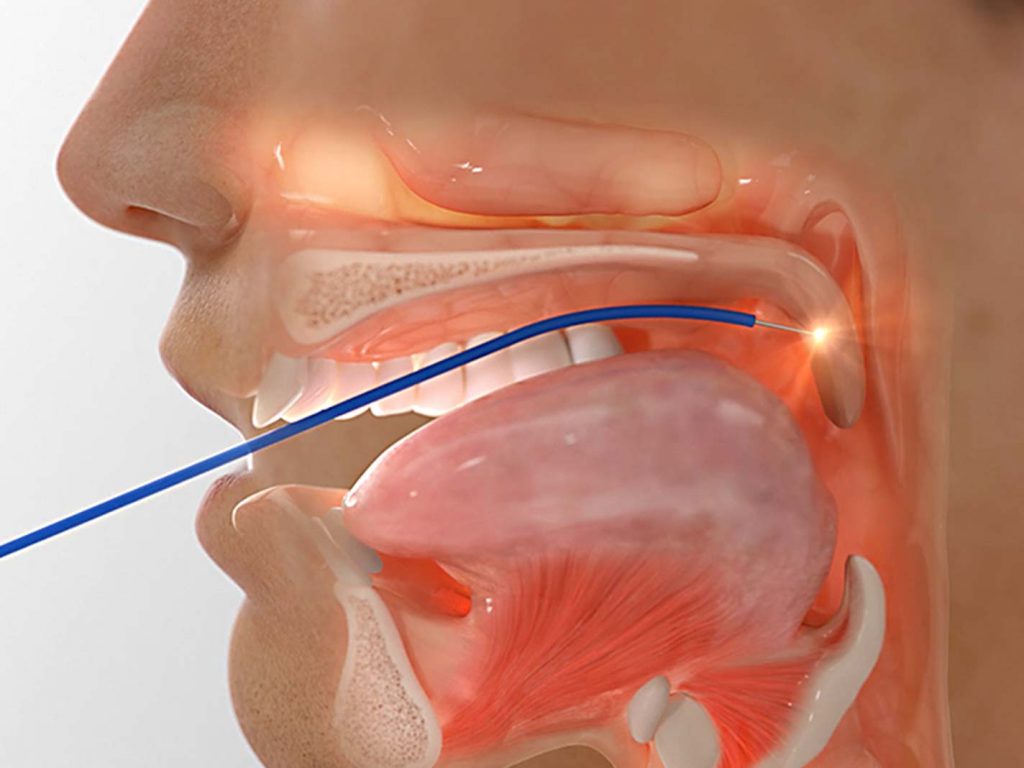
NMES physiologically retrains the upper airway and tongue, to stay in its natural position while you sleep.
eXciteOSA For Sleep Apnea – A Daytime Therapy
Neuromuscular electrical stimulation (NMES) with eXciteOSA®
NMES safely activates nerves and trains muscles. NMES is a familiar and well-established technique in medicine and athletics.
Sleep apnea has multiple causes, including reduced responsiveness of the tongue and upper airway muscles4.
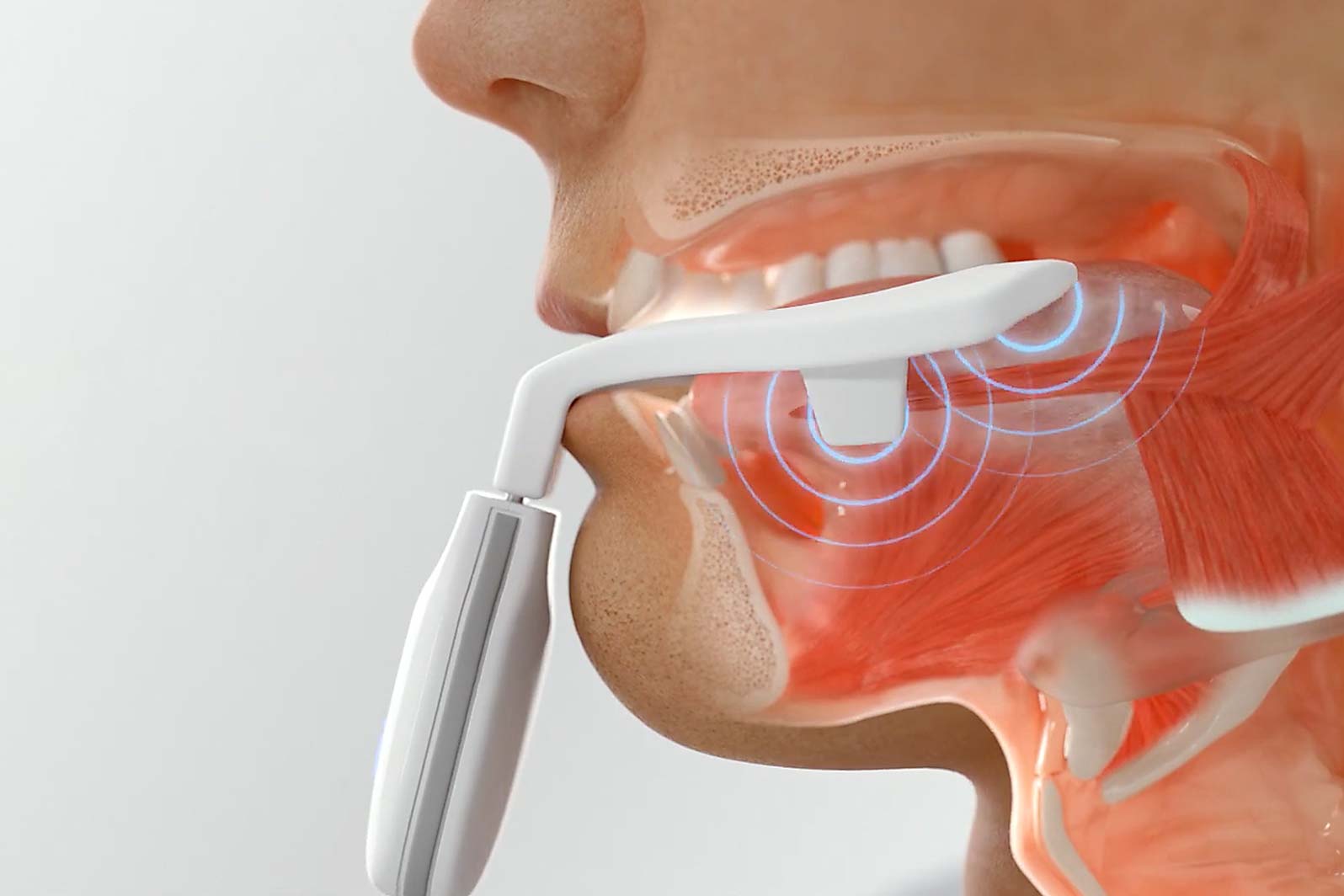
eXciteOSA for sleep apnea changes this. It trains your tongue to stay in position, letting you sleep soundly.
 NMES (eXciteOSA®) |
 MAD / CPAP |
 SURGERY |
|
| Treats a root cause of snoring |
|
|
|
| Nothing to wear at night |
|
|
|
| Evidence based |
|
|
|
| Low cost trial period |
|
|
|
| Helps your body to help itself |
|
|
|
NMES is different
Convenient daytime therapy
Comfortable and painless leaving you with nothing to wear at night.
Reduces Apneas
A recent study found a 52% reduction in the AHI and a 50% reduction in the ODI amongst responders – all without a CPAP or MAD3.
Retrains the upper airway
Physiologically retrains the upper airway. No other device currently on the market does that.
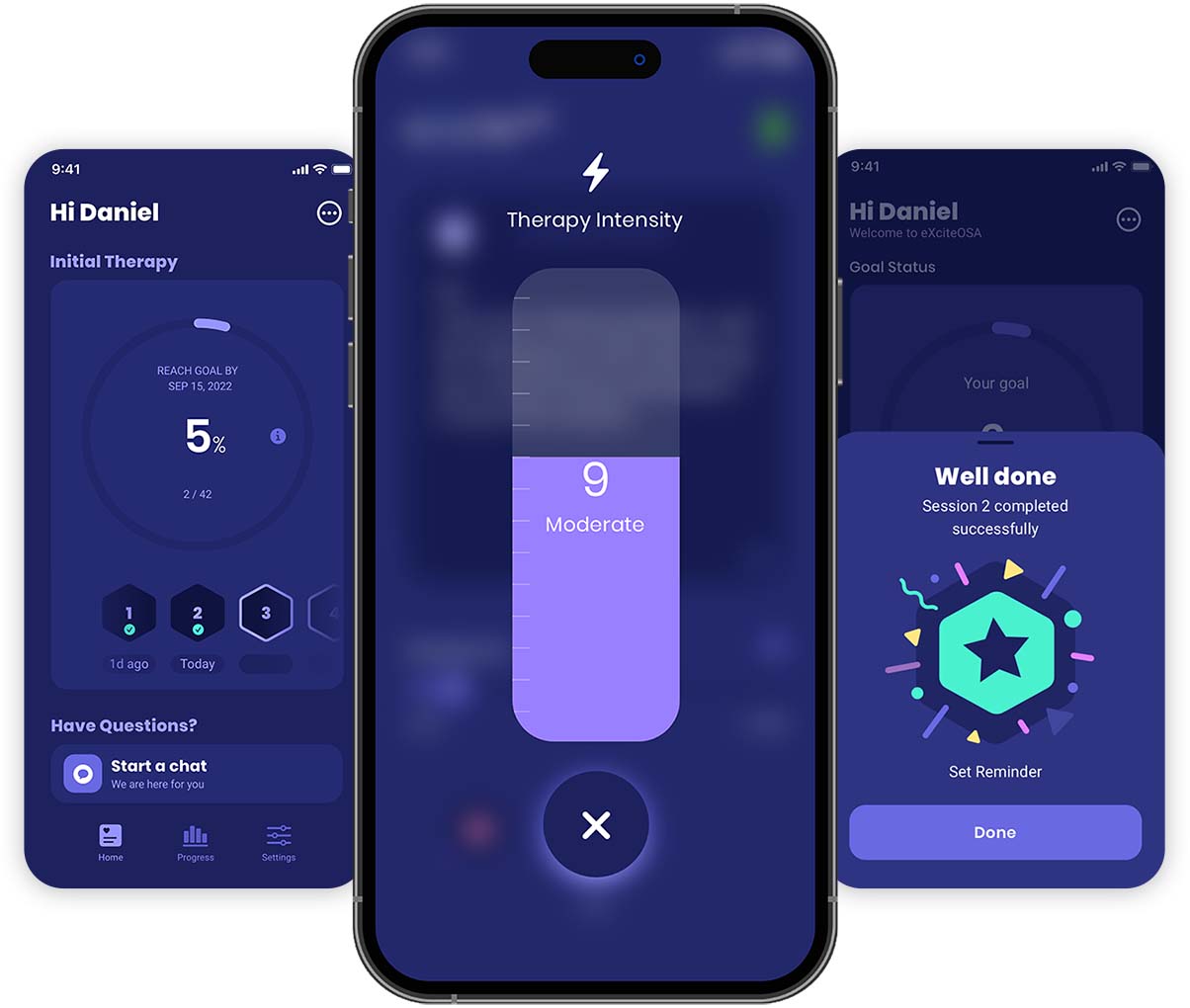
The App
You are in control
Use the app to control the intensity of the stimulation and get guidance, reminders and notifications about your therapy.

Better sleep or your money back.*
If you do not experience improved sleep after completing 70% of 42 therapies in 8 weeks, we will refund you the price of the device.
Hear how eXciteOSA® has transformed people’s sleep
“I have this simple device… [that] I use during the day [so] that I can sleep quite happily without thinking about having to wear a mask at night.”
Find a Therapy That Works for You
OSA is a progressive disease that should be treated5-8
With new alternatives available, like daytime therapy, there is now no excuse. Consult a physician as, if left untreated, OSA places extra strain on your health.
Daytime therapy. No nighttime wearable.
Clinically proven to reduce mild OSA in just 6 weeks
Frequently asked questions
Transcutaneous electrical nerve stimulation (TENS) machines stimulates the sensory nerves (the nerves that send signals from the body to the brain) with the purpose of disrupting the pain signal.
Neuromuscular electrical stimulation (NMES) also uses electrical stimulation, but targets the motor nerves (the nerves that send signals from the brain to the body) in order to stimulate the muscles directly.
Sensory and motor nerves fire at different frequencies, which is how TENS and NMES devices are able to impact them differently.
eXciteOSA® is a user-controllable neuromuscular electrical stimulator (NMES) that delivers a mild electrical current with defined frequencies to stimulate and improve muscle function in the mouth and tongue.
Unlike traditional sleep apnea treatments, eXciteOSA® targets the tongue to promote endurance of the muscle, thereby reducing airway collapse and snoring during sleep.
A daytime therapy with no night-time wearable necessary for a better night’s sleep.
There are several options for treating sleep apnea.
Manudibular advancement devices (MADs) work by temporarily moving the jaw forward, in order to create more space in the airway.
CPAP delivers air pressure through a mask to open up the airway.
Surgery is usually an option to consider after other treatments have failed. Examples include removal of soft tissues from the airway, or procedures designed to stiffen the airway.
NMES physiologically retrains the upper airway and tongue to maintain the tongue’s natural position while you sleep, effectively helping your body help itself. eXciteOSA for sleep apnea is the only device that delivers NMES. You can also refer to the chart above to better illustrate the main difference.
Risk-free trial
*The eXciteOSA Better Sleep Guarantee: Consistent use is critical to success. While we don’t expect perfection, we do ask that you complete 70% of your 42 therapies within 8 weeks of receiving your product before receiving your refund. Refunds are available to customers who purchased eXciteOSA without using covered benefits or had the device reimbursed under any type of health insurance.
A session is equivalent to 20 minutes. In addition, it is recommended that you increase the therapy intensity gradually over the treatment period to ensure that you give the treatment every chance of being effective. Please note that once the initial therapy is finished and to maintain the treatment you need to use the device at least twice a week forever.
The mouthpiece is a precision piece of engineering responsible for delivering neuromuscular electrical stimulation targeted primarily at the tongue. It needs to be replaced every 3 months.
References
- Kotecha B, Wong PY, Zhang H, Hassaan A. A novel intraoral neuromuscular stimulation device for treating sleep-disordered breathing. Sleep Breath 2021;25(4):2083-2090.
- Baptista PM, Martinez Ruiz de Apodaca P, Carrasco M, Fernandez S, Wong PY, Zhang H, Hassaan A, Kotecha B. Daytime neuromuscular electrical therapy of tongue muscles in improving snoring in individuals with primary snoring and mild obstructive sleep apnea. J Clin Med 2021;10(9):1-11.
- Nokes B, Baptista PM, Martínez Ruiz de Apodaca P, Carrasco-Llatas M, Fernandez S, Kotecha B, Wong PY, Zhang H, Hassaan A, Malhotra A. Transoral awake state neuromuscular therapy for mild obstructive sleep apnea. Sleep Breath [accepted; in-press].
- Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea: Identification of novel therapeutic targets. Am J Respir Crit Care Med 2013;188(8):996-1004.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015-3021.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217-1239.
- Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 2005;165:2408-2413.
- Redline S, Schluchter MD, Larkin EK, Tishler PV. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep 2003;26:703-709.
- Vgontzas AN, Li Y, He F, Fernandez-Mendoza J, Gaines J, Liao D, Basta M, Bixler EO. Mild-to-moderate sleep apnea is associated with incident hypertension: age effect. Sleep 2019;42(4):zsy265.
- Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 2005;172(12):1590-1595.
- Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastiao YV, Wen Y, Schwartz S, Borenstein AR, Wu Y, Morgan D, Anderson WM. Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep 2017;40(1):zsw032.