
Treat the cause, not the symptoms
The first and only noninvasive device for sleep apnea that treats a root cause, not the symptoms. In 20 minutes a day. Not 8 hours a night.
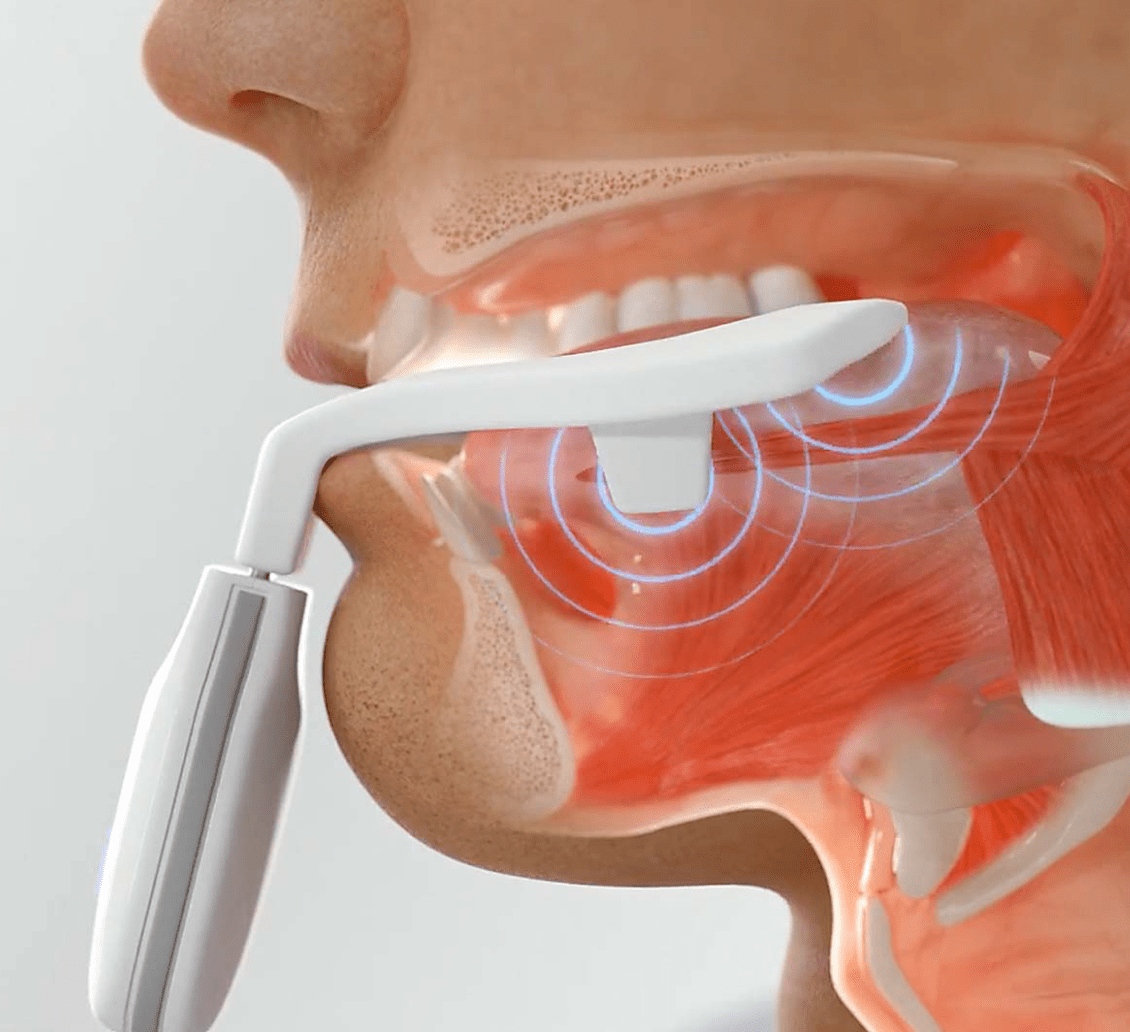
The mouthpiece will need replacing every 90 days from first use. This is to ensure the performance and safety of the mouthpiece meets recognized international standards.
Clinically Proven
Multiple clinical studies with 100s of patients conducted by prestigious universities and more on the way1-4.
FDA authorized
The first-ever FDA-authorized daytime treatment for mild obstructive sleep apnea.
Patient endorsed
More than 10k eXciteOSA product activations and over 750k therapy sessions completed.
Trusted by physicians
100’s of sleep physicians are recommending and prescribing eXciteOSA around the world.

My name is James and I will be your Sleep Advocate during your eXciteOSA treatment program.
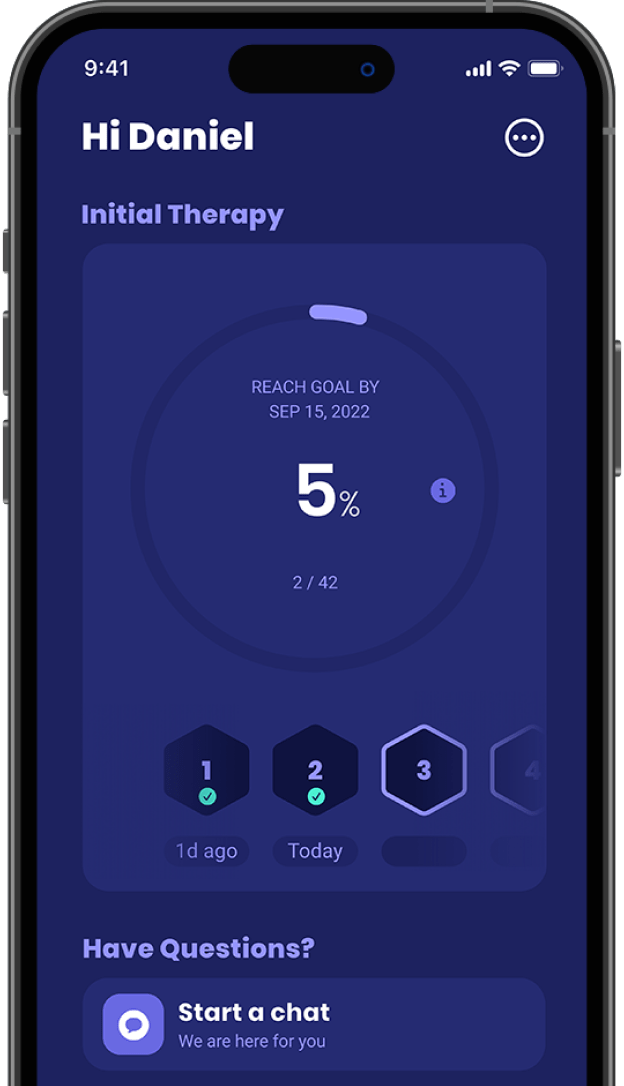
Your therapy journey
Delivered by eXciteOSA, controlled by the app and supported by our sleep advocate team.
Backed by evidence
Over 140 patients in multiple studies and more on the way, we’re committed to the science.

Daytime therapy
Noninvasive daytime treatment. Use for 20 minutes a day, not 8 hours a night.

Compact and portable
Portable and easy allowing you to self-administer treatment virtually anywhere at any time.

No nighttime wearables
No surgery or nighttime wearables. Sleep well breathing normally and naturally all night.
Happy sleepers
With over 10,000 patients on therapy, real-world experience, and clinical studies adherence over 80%, hear why patients choose eXciteOSA for their sleep apnea therapy.
Proud to be supporting our military and Veterans
Learn more about eXciteOSA and how it’s helping thousands of Veterans get a better night’s sleep, for just 20 minutes a day with no nighttime wearable.
Trusted by experts
Backed by leading sleep specialists
The mouthpiece will need replacing every 90 days from first use. This is to ensure the performance and safety of the mouthpiece meets recognized international standards.
References
- Wessolleck E, Bernd E, Dockter S, Lang S, Sama A, Stuck BA. Intraoral electrical muscle stimulation in the treatment of snoring. Somnologie 2018;22(2):47-52.
- Kotecha B, Wong PY, Zhang H, Hassaan A. A novel intraoral neuromuscular stimulation device for treating sleep-disordered breathing. Sleep Breath 2021;25(4):2083-2090.
- Baptista PM, Martinez Ruiz de Apodaca P, Carrasco M, Fernandez S, Wong PY, Zhang H, Hassaan A, Kotecha B. Daytime neuromuscular electrical therapy of tongue muscles in improving snoring in individuals with primary snoring and mild obstructive sleep apnea. J Clin Med 2021;10(9):1-11.
- Nokes B, Baptista PM, Martínez Ruiz de Apodaca P, Carrasco-Llatas M, Fernandez S, Kotecha B, Wong PY, Zhang H, Hassaan A, Malhotra A. Transoral awake state neuromuscular therapy for mild obstructive sleep apnea. Sleep Breath [accepted; in-press].











