Committed to the science
Founded by medical professionals, we specialize in developing creative and innovative science-based products that directly improve patient’s lives.
Tried, tested and proven – Hear what doctors and patients think.
Backed by evidence
Multiple clinical studies, conducted by prestigious universities with nearly 150 patients have consistently demonstrated the efficacy of eXciteOSA.
Supported by doctors
Prominent sleep physicians around the world recommend eXciteOSA to their patients for mild sleep apnea or snoring.
Loved by patients
Because eXciteOSA is only used 20 min during the day, it is easy to stick to therapy.
Backed by evidence
Over 140 patients in multiple studies and more on the way, we’re committed to the science.
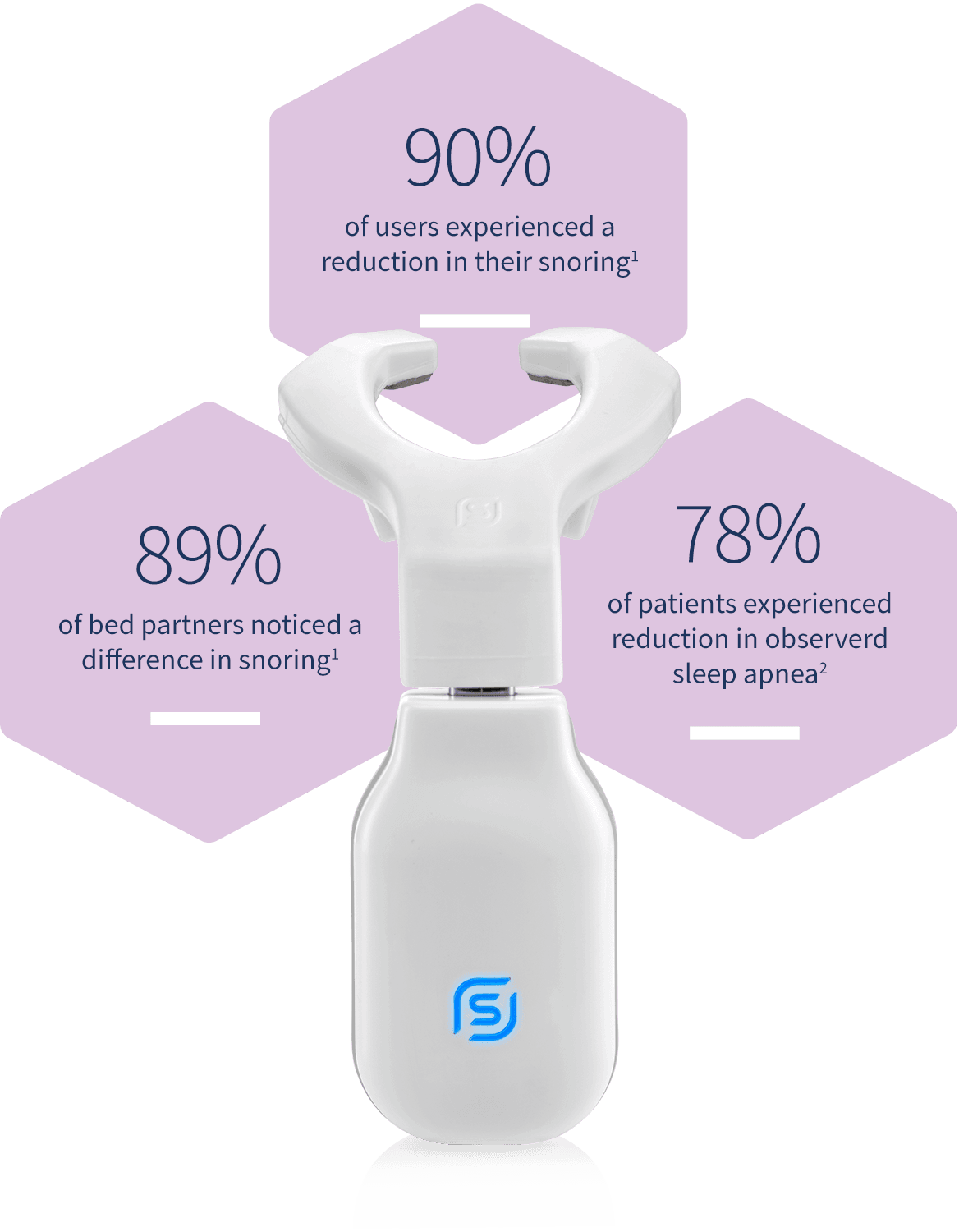
Proven reduction in snoring
Reduction in snoring was assessed in a clinical study of 115 patients. Snoring levels were measured with a microphone equipped with a snore sensor and placed on the patient’s chest. The measurements were taken pre and post-therapy.
The results indicated that 90% of patients experienced a reduction in total time spent snoring above 40dB. 40dB is the threshold that the WHO uses to define night-time noise pollution1.
External link
Bed partners witnessed improvement
In the same clinical study, patients’ bed partners were asked to rate the level of snoring on a scale before and after therapy. 89% of partners rated the snoring as noticeably lower after 6 weeks of therapy with eXciteOSA1.
External link
Reduction in sleep apnea
In a study with 65 sleep apnea patients, a 2-night home sleep test pre and post-therapy measured objective improvement in sleep apnea. The test results showed a significant reduction in apnea-hypopnea index (AHI) and oxygen desaturation index (ODI) measures2.
External link
Supported by doctors
Loved by patients
I would recommend this device to anyone with sleep apnea.
It can be used anytime of day for 20 minutes without bothering me when I’m trying to sleep.
It is indeed a life changing device
I’ve been using eXciteOSA for the last 3 months and I must say it really helps improving the quality of your sleeping. I wake up more energised and I don’t need CPAP anymore. The therapy is easy to do, you can do it while watching a film or TV.
eXciteOSA has been a relief for my wife and me!
She awakens refreshed and energetic- not groggy as in the past. I also benefit as I am not woken up multiple times during the night! I absolutely can appreciate a change in her energy level in the mornings and she doesn’t become so exhausted at the end of the day.
I have been so pleasantly surprised
I’ve gone through so much with my overall health and wasn’t sure if this would help me. IT DID! I don’t wake up feeling groggy or depleted. I feel like my weight loss regimen is working now since I’m getting recuperative sleep.
I am very grateful for eXciteOSA.
Now that I have been using the eXciteOSA I enjoy 5-6 hours of straight uninterrupted sleep and I feel better during the day with little tiredness.
Highly recommend it
I was diagnosed with mild sleep apnea. I used to have attacks at night with the feeling of not breathing, some attacks very scary. eXciteOSA was a big positive change for me. I don’t experience these attacks anymore and sleep much better.
References
- Baptista PM, Martinez Ruiz de Apodaca P, Carrasco M, Fernandez S, Wong PY, Zhang H, Hassaan A, Kotecha B. Daytime neuromuscular electrical therapy of tongue muscles in improving snoring in individuals with primary snoring and mild obstructive sleep apnea. J Clin Med 10(9):1-11 (2021).
- Nokes B, Baptista PM, Martínez Ruiz de Apodaca P, Carrasco-Llatas M, Fernandez S, Kotecha B, Wong PY, Zhang H, Hassaan A, Malhotra A. Transoral awake state neuromuscular stimulation therapy for mild obstructive sleep apnea. Sleep & Breathing (in-press; 2022).









